
9 October 2025
ubispoke® - We make the molecules you need
Supply Chain Experts. We manufacture the molecules you need.
Unlock new possibilities with Ubichem. With over 50 years of expertise in chemical synthesis, ubispoke® recently launched as the leading service for custom synthesis. A specific focus is the supply and synthesis of key starting materials (KSM) for the development of small molecule new chemical entities in clinical drug discovery. Partner with us to elevate your projects and drive innovation in pharmaceutical development.
Understanding that seamless supply chain logistics are essential for your success, we're here to mitigate supply chain disruptions by offering diverse geographical options for sourcing raw materials, with manufacturing sites in China, India, and Europe. With ubispoke®, you can trust that we'll deliver the molecules you need - when you need them. Our unrivalled manufacturing capabilities span a wide range of synthetic methodologies, providing scalability from grams to commercial volumes for even the most challenging, commercially inaccessible molecules.
ubispoke® offers flexible manufacturing options, including both non-GMP and GMP solutions, which enables us to meet an array of synthetic needs across the most complex chemistries.
With our vast market insights and expertise in navigating intricate regulatory compliance, we deliver comprehensive solutions that minimise the risks associated with supply chain disruptions. Customers operate with confidence, enjoying peace of mind knowing their supply chain is securely managed by our dedicated team.
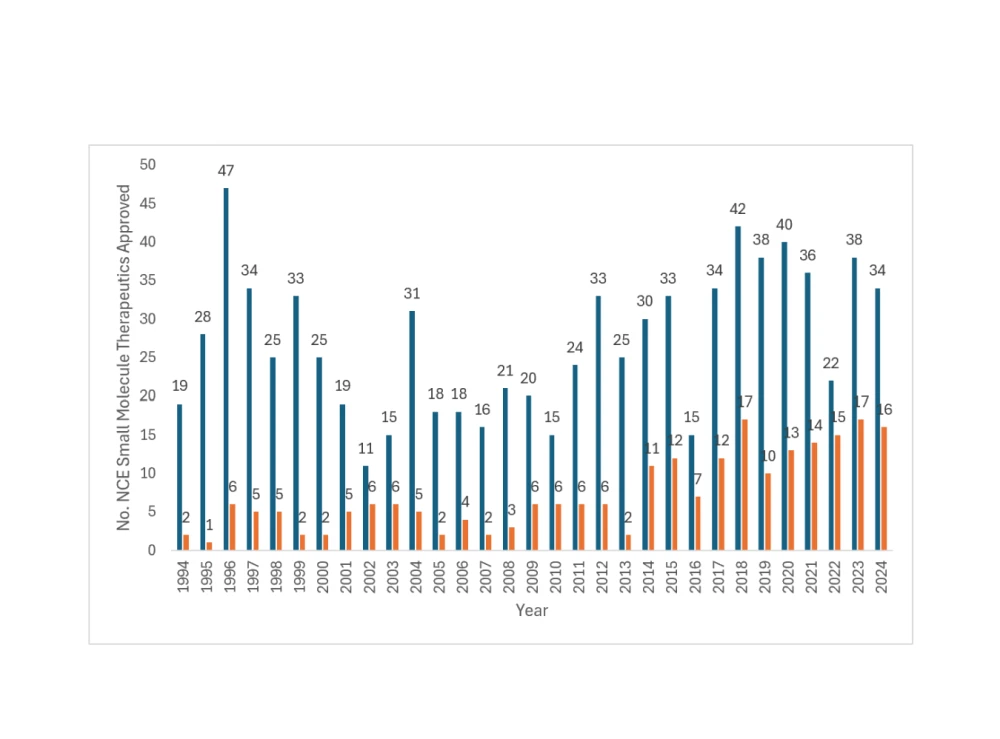
Small molecules continue to dominate drug pipelines
Small molecule therapeutics continue to make up the lion’s share of FDA drug approvals, despite the increasing interest in peptides and oligonucleotides. A recent 10-year survey by CDER reveals an average of 47 small molecule drug approvals annually. In 2024 alone, 31 out of the 50 FDA-approved drugs were innovative small molecule new chemical entities (Wang et al., 2015).
ubispoke® is your trusted solution in the manufacturing and supply of nitrogen heterocycles, aromatic compounds, fluorinated materials, and chiral building blocks essential for the forward synthesis of small molecule therapeutic drugs.
Developing equity in pharmaceutical innovators and CDMOs around the globe, establishes robust, reliable, and cost-effective supply chains for critical key starting materials (KSMs). These KSMs are vital in the early stages of synthesis for small molecule drugs entering Phases I, II, and III of clinical testing, paving the way for new drug application (NDA) approvals.
For example, ubispoke® provides a diverse range of KSMs in volumes from 1 kg to 1 tonne.
ubispoke® offers expertise in asymmetric synthesis, fluorination, and heterocyclic chemistry and positions Ubichem as your trusted partner for delivering innovative and commercially viable solutions. We enhance your process by increasing yields, improving the economic viability of routes of synthesis and promoting greener chemistries.
Recognising the significance of these chemistries is essential for thriving in today's drug discovery landscape. In 2024, from the FDA approved drugs, 84% contained a heterocyclic skeleton, 61% had at least 1 chiral centre and 35% included a fluorine moiety. These insights, backed by compelling data, underscore our deep understanding and commitment to advancing modern drug discovery.

ubispoke®: The Preferred Partner for Success
Dependability and Compliance: Experience the peace of mind that comes from our robust supply chains and strict regulatory adherence, ensuring seamless operations at every step
Advanced Capabilities: Leverage our trusted manufacturing facilities that provide a comprehensive range of chemical expertise tailored to your needs
Scalability and Efficiency: Experience seamless production transitions from small-scale to bulk manufacturing, designed to meet your evolving demands
Global Regulatory Proficiency: Rest easy knowing that our compliance with ECOVADIS, ISO 9001, and GMP, along with our expertise in handling REACH requirements, opens the door to international markets for your products
Harnessing the Power of Collaboration: Together, we deliver commercially viable solutions that empower pharmaceutical innovators and biopharmaceutical companies worldwide
Experts for Experts: Our team consists of experts from the chemical industry: process, medicinal, analytical and formulation chemists
Open the Door to Innovation:
ubispoke® expertise interprets the intricacies of your needs and has the power to bring together the right partners. With our unique and compelling technical propositions, we are the collaborator of choice for customers across the globe.
Connect with ubispoke® today and embark on a journey towards innovative solutions that drive your success.
References:
Wang, Y., Yang, F., Wang, B., Xie, L. and Chen, W., 2025. New FDA drug approvals for 2024: Synthesis and clinical application. European Journal of Medicinal Chemistry, p.117241.
Seoane-Vazquez, E., Rodriguez-Monguio, R. and Powers III, J.H., 2024. Analysis of US Food and Drug Administration new drug and biologic approvals, regulatory pathways, and review times, 1980–2022. Scientific reports, 14(1), p.3325.


